Back to the Lab: Successes and failures in diagnosing COVID-19
By Seth Young, B.S.
Mr. Young is an undergraduate student double-majoring in Chemistry and Biology at Emory University in Atlanta, GA, USA. In addition to working as a research assistant in Dr. Dennis Liotta’s lab, Mr. Young is also a nationally certified emergency medicine technician.
As the coronavirus pandemic continues, laboratory diagnostic techniques have garnered increasing public attention and scrutiny. In my last article, I reviewed the basics of diagnostic PCR and antibody testing for SARS-CoV-2. However, the ability to perform PCR and other laboratory tests is not enough to enable efficient diagnosis. Particularly during a pandemic, laboratories must have a strategy for handling many samples at a time. In this article, I discuss one such method, called “gridding” or “pooling”.
What is Group Sampling?
Group (or batch) testing is a technique to enhance laboratory efficiency by combining samples from several individuals, instead of testing each of their samples individually. It was first proposed by Robert Dorfman in 1943 as a method to test for syphilis [1]. In its simplest form, samples from multiple specimens are combined into a group sample so that only one test needs to be run on the entire group. If a group sample tests negative, the lab technician can continue to the next group sample. If it tests positive, however, then new samples from each specimen in the positive group must be tested individually. For example, you could combine 90 specimens into 10 different groups, each with samples from nine specimens. You then run your test on the 10 groups. If one of your 10 groups tests positive, you then run individual tests on the nine specimens in that group. This decreases the total number of tests needed from 90 to 19.
A Nature news article recently described three slightly more complex methods of group testing. In the second method, if a group sample tests positive, new samples from each specimen are regrouped into smaller batches. These new smaller group samples are retested. Individual tests are run only on the specimens in the small group sample that tests positive. Let’s re-examine the example for the first method. You again group 90 specimens into 10 different groups and run your tests on those 10 group samples. If one group tests positive, you regroup the nine potentially positive specimens into three groups of three specimens each. You run three more tests, narrowing your positive group sample to only three specimens that must have individual tests. This reduces the number of tests needed from 90 to 16.
Figure 1. Reagents for COVID-19 RT-PCR Panel. This image was made freely available by Wikimedia.
The more complex methods use matrices or “grids” to identify positive samples within groups. In one type of grid testing, once a group specimen tests positive, the involved specimens are regrouped. Instead of being grouped into three groups of three, however, they are grouped into six groups of three, with each specimen contributing a sample to two groups (see Figure 1). When the six small groups are tested, two of these will test positive. The positive specimen can be identified as it will be the only specimen present in both positive groups. This again reduced the number of tests in our example from 90 to 16; however, with larger numbers of samples, the number of tests can be reduced even further.
Another grid strategy uses only one round of testing by dividing all specimens into multiple group samples, with each specimen represented in multiple groups from the start. Then, when several groups test positive, the laboratory technician can use logic to deduce which specimen must be the positive one based on the pattern of specimens represented per group. The elaborate mathematics required to do this effectively can lead to error and even slow down testing. However, such error can be mitigated though through use of computationally generated groups.
Figure 2. Grid Testing. In this example, black circles represent negative specimens, and the red circle represents the positive specimen. This group sample of nine specimens has tested positive due to the one positive specimen. These nine samples are retested in six groups of three samples each, with each test being represented by either a column or a row. Both test #3 and test #5 will result positive. Since the red circle is the only specimen represented in both positive tests, we can deduce that it must be the positive specimen. Image created by Mr. Young.
Barriers in Diagnosis
Not all diagnostic strategies have panned out. Of course, the least effective diagnostic strategy is not testing at all. Testing to track and trace, quarantining, appropriately using PPE, and continuing personal practices such as social distancing and hand washing are currently the only definitive tools we have to prevent spread of this disease.
One diagnostic barrier has been kit contamination with positive control material. Whenever a test is run, a guaranteed “positive” specimen must be present to ensure the test is working properly. In the case of COVID-19 PCR, the positive specimen contains known genetic material of SARS-CoV-2. During the efforts to scale up mass production quickly, positive specimens cross-contaminated other portions of kits and caused problems with testing accuracy. The contamination resulted in false positives, leading to false scares and requiring re-testing. Speed is certainly a critical factor in this pandemic, but not at the cost of accuracy.
Figure 3. Pyxis Robot assisting with medication delivery. Technologies like this robot help reduce the strain on the medical system and reduce risk of disease spread. Image available through Wikimedia.
Diagnostic testing also still requires a certain degree of human-human interaction, putting health care workers and laboratory technicians at risk of contracting or spreading COVID-19. Rwanda has begun using robotic assistants to test patients and administer medications. This effectively reduces the amount of contact medical staff have with potentially infected patients, reducing the strain on the medical system. However, the most important ways to mitigate infection in the healthcare are appropriate use of PPE and ensuring biosafety measures continue in the laboratory
Several novel diagnostic efforts are in the works. A research team at the Pasteur Institute in Dakar, Senegal is reportedly developing a quick and cheap test for COVID19. According to news reports, the test is designed similar to a pregnancy test, and indeed the Pasteur Institute has partnered with Mologic, a British company and producer of one of the first home pregnancy tests. A small amount of biological sample is added to a stick-like device which shows a line when one is infected with COVID19. While the researchers have not released details of the test, they are likely measuring the virus’ S-protein or antibodies directly from the samples. This rapid test remains pre-production, as progress was hampered when several staff members at the Pasteur Institute become infected with SARS-CoV-2 this June.
The German company Pharmact AG has developed a similar rapid point-of-care test that takes 20 minutes to determine the presence of coronavirus immunoglobulins. Unfortunately, the German test costs ~40 Euros, affordable only to the wealthy. Nonetheless, African scientists are continuing the work to develop a cheaper method. In Ghana, for example, Incas Diagnostics aims to scale production of a cheap antibody test. Ideally, this will decrease the need to import expensive tests and it will help identify disease spread in lower-resource areas. However, the diagnostic accuracy of many of these tests remains unproven [2].
Figure 4. German rapid antibody test from Leibniz Institute of Photonic Technology (Leibniz IPHT) at the InfectoGnostics research campus. Image made publicly available by U.S. Army Europe.
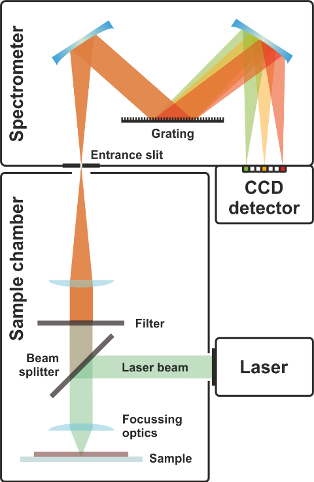
Other proposed forms of testing center around avoiding using various chemical and biological reagents, instead opting to use electric and optical methods to detect the virus. Techniques such as Raman spectroscopy take advantage of particle properties such as size and composition [3]. COVID-19 has unique electrical properties due the composition of its shell. When particles of COVID-19 are suspended in water, they can scatter light in a distinct fashion allowing for easy detection. Most of the work and research on this involves properly determining which kinds of light are affected and to what extent. There are many other molecules in patient samples that must be filtered out as noise. These detection methods lend themselves well to wide public screening, such as detecting virus particles in human waste samples from sewage treatment or other public areas.
Figure 5. Raman spectroscopy. Raman spectroscopy is a chemical analysis technique that may help in COVID-19 diagnosis. It provides information about particles by examining the way particles scatter light. This image was made available by Wikimedia.
Finally, the many exciting news reports contrast with the relative paucity of peer reviewed research to support them. During this pandemic, companies have gained some leniency to meet demand for new diagnostics. The WHO, the US FDA, and some African regulators have permitted expedited approval of certain COVID-19 tests. Such permissions are essential to enable more testing globally. At the same time, as consumers, we should remember the potential differences in sensitivity and specificity of expedited testing methods when making diagnostic or public health decisions to combat the virus.
Mr. Young has no financial involvement or interest in the Bio Africa Marketplace or products mentioned therein or elsewhere within the BioAfrica Innovation Hub websites. His role in the Scientific Resource Hub does not constitute endorsement or recommendation of specific products or suppliers mentioned within the Bio Africa Innovation Hub websites.
References
Publicly available websites and news articles are linked in the text. Full references for peer-reviewed articles or other sites not publicly accessible are available below.
1. Dorfman, R., The Detection of Defective Members of Large Populations. Annals of Mathematical Statistics, 1943. 14: p. 436-440.
2. Lisboa Bastos, M., et al., Diagnostic accuracy of serological tests for covid-19: systematic review and meta-analysis. BMJ, 2020. 370: p. m2516.
3. Khan, R.S. and I.U. Rehman, Spectroscopy as a tool for detection and monitoring of Coronavirus (COVID-19). Expert Rev Mol Diagn, 2020. 20(7): p. 647-649.